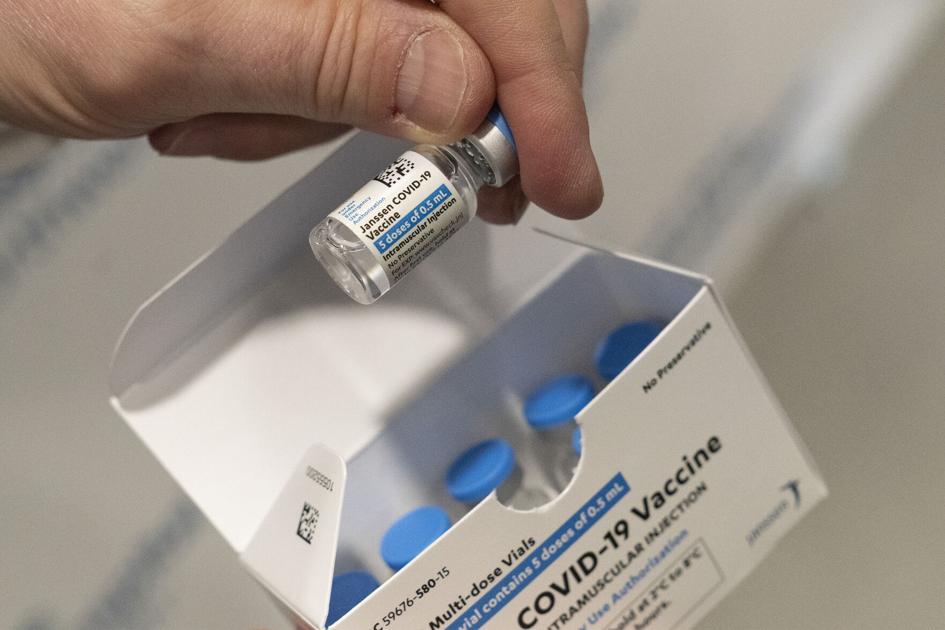
The Baltimore plant contracted to manufacture Johnson & Johnson’s COVID-19 vaccine was dirty, did not follow production protocols and its staff was not trained, resulting in contamination of the injection material, they revealed on Wednesday.
Food and Drug Administration (FDA) has released a report on the recent inspection of the Emergent Biosciences plant, whose production has been stopped.
Inspectors said a batch of substances used for the J&J vaccine, which requires a single injection, was contaminated with material used for Covid-19 vaccines from another J&J customer, AstraZeneca. The entire batch had to be discarded, which the report says is enough to make about 15 million doses of J&V vaccine.

The European Medicines Agency has found a “possible link” between the vaccine and the very rare cases of bleeding disorders detected in the United States
The report mentions other problems, such as peeling paint, black and brown residue on floors and walls, poor cleaning and non-compliant employees to prevent contamination.
Emergent and Johnson & Johnson said on Wednesday that they are trying to solve the problems as quickly as possible. To date, none of the products manufactured there for J&J have been distributed.
The use of the J&J vaccine is currently suspended in the United States because authorities are investigating its connection to certain very rare blood clots. Almost 8 million doses of this vaccine from Europe have been used.

WASHINGTON – The United States will take steps to resume the application of the covid-19 vaccine …
The Baltimore plant shut down production late last week at the request of the FDA. The Agency did not grant the factory the necessary emergency authorization for the distribution of its products.
The FDA said the substance produced by Emergent is being stored pending further testing.