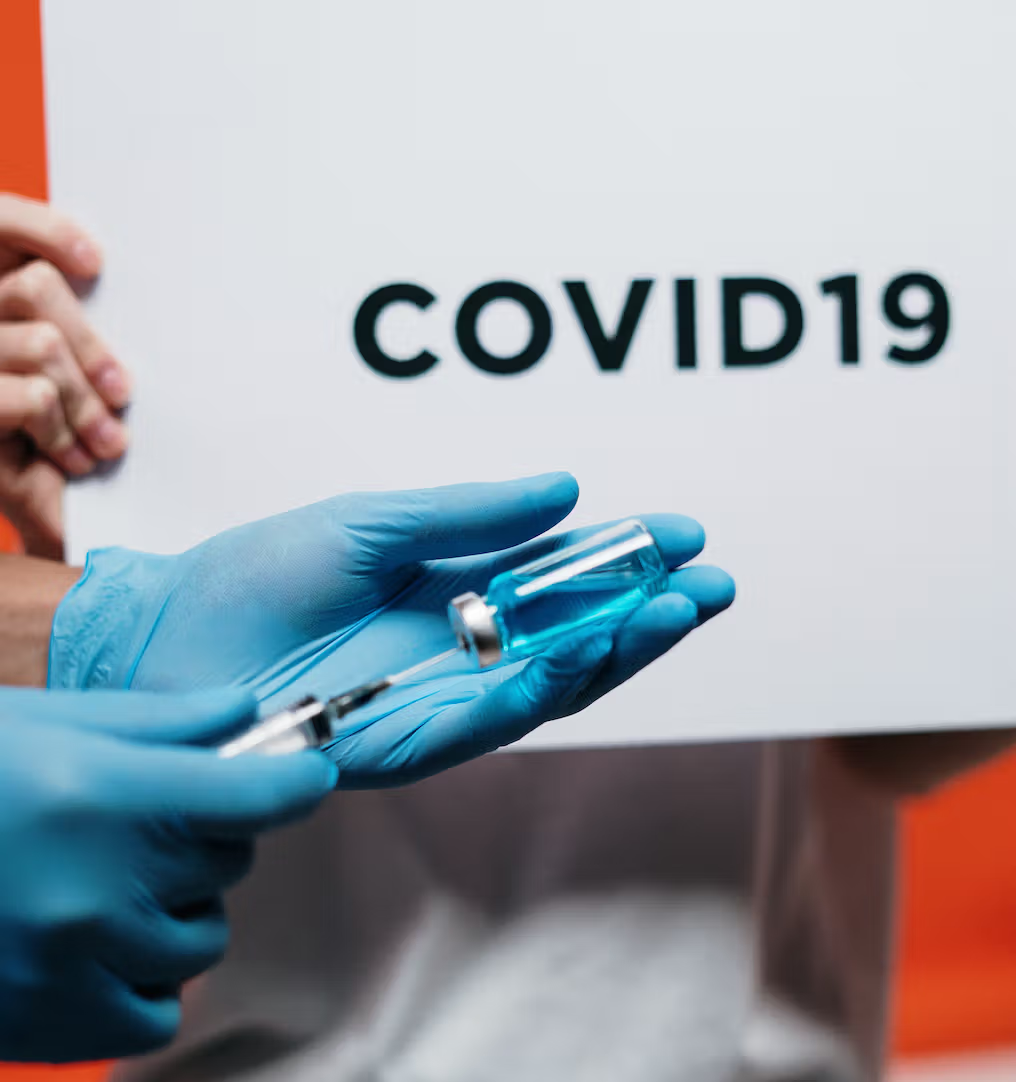
Aware that Pfizer and BioNtech COVID-19 vaccine vials may provide an additional dose, the federal agency has updated the labeling to allow the use of low-volume syringes to extract it.
The US Food and Drug Administration (FDA) has approved an updated label that allows a potential additional dose of Pfizer and BioNtech COVID-19 vaccine to be extracted using low-volume syringes, as reported on CNN.
“Syringes and / or needles with a low dead volume may be used to extract six doses from a single vial. If standard syringes and needles are used, there may not be enough volume to extract the sixth dose from a single vial. bottle, “the FDA said.
While overfilling the Pfizer and BioNtech COVID-19 vaccine vials may provide an extra dose, low-volume syringes are not standard and many hospitals, medical centers and vaccine dispensaries may not have access to them right now.
It has been reported in recent days that Pfizer plans to count the additional doses for its commitment of 200 million doses distributed in the US by the end of July this year.
This decision, originally in a story from New York Times, comes at a time when the supply of vaccines is extremely necessary and the launch of the vaccine has been slower than expected. And, some health care providers say that the extra doses are not in line with every ampoule.
In the same story, it was also reported that Pfizer executives challenged the formulation of the emergency vaccination authorization to recognize the dispensed vials containing 6 doses, not 5. The difference, recognized by the FDA on January 6, influences the pharmaceutical company’s contract. with the US government.
This can also make it a challenge for the new administration to achieve its vaccination goal. President Joe Biden has set a goal of administering 100 million doses of vaccine in the first 100 days of administration.