Dragons lurk at the edges of the map of known elements – atomic giants so delicate and so rare that they defy easy study.
Such a behemoth eventually gave up at least some of its secrets, with chemists managing to gather enough einsteinium to materialize important details about the chemistry and ability of the mysterious element to form bonds.
For more than 70 years, the isotopes of einstein have proved frustratingly difficult to study. Either they are too difficult to achieve, or they have a half-life of less than a year, and what is precious is created and begins to fall apart like a sandcastle at tide.
The behavior of the element is supposed to follow the patterns of its less robust colleagues in the actinide series. This is clear. But due to its size, the strange relativistic effects make it more difficult to predict how it will react in certain chemical processes.
Usually, such confusion is easily clarified by simply conducting a series of experiments.
The Lawrence Berkeley National Laboratory of the US Department of Energy has finally gathered enough of all things to do just that.
More informally called the Berkeley Lab, the famous institute is already responsible for discovering a significant chunk of the upper limits of the periodic table of elements.
A dozen of them were the work of nuclear physicist Albert Ghiorso, a lifelong Berkeley researcher whose early career saw him develop radiation detectors as part of the Manhattan Project.
In the early 1950s, Ghiorso detected weak traces of two as yet unidentified radioactive elements in the airborne dust collected by aircraft flying after the first full-scale test of a thermonuclear device.
One of these elements was later called einsteinium, named after none other than the famous theorist born in Germany.
With an atomic mass of 252 and containing 99 protons, it is not easy. As with all transuranic elements – elements heavier than uranium – einsteinium requires serious physics to produce.
There is no convenient source or deposit to dive into. Cooking a batch requires shooting smaller relatives, such as curiosity, with a bunch of neutrons in a nuclear reactor and then having a lot of patience.
The early efforts of the 1960s produced just enough to see with the naked eye, weighing in at a tiny 10 nanograms. Subsequent attempts did a little better, although they led mainly to impure series.
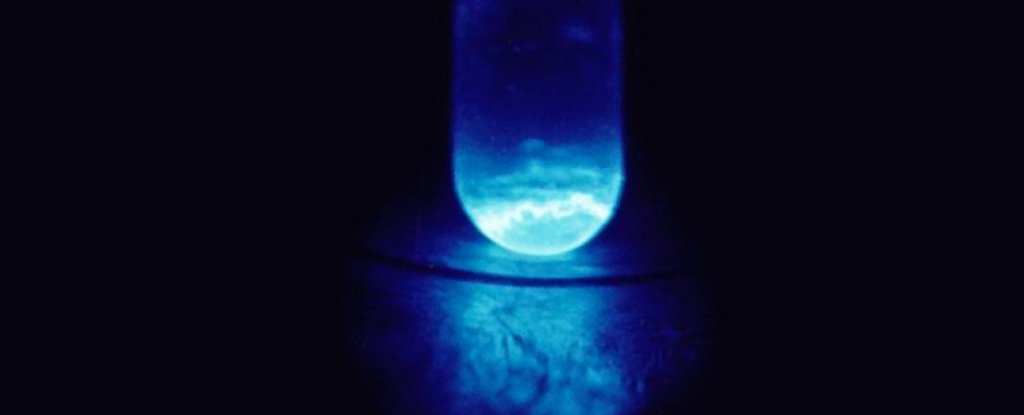
This time, the researchers came up with about 200 nanograms of the isotope Einsteinium E-254, framed as part of a complex with a carbon-based molecule called hydroxypyridinone.
Getting here was not easy, affected by the contamination of the smaller elements and the inevitable impact of the average pandemic closure – just the thing that threatens an experiment dependent on a rapidly decaying material.
“It is a remarkable achievement that we have been able to work with this small amount of material and make inorganic chemistry,” says researcher Rebecca Abergel.
“It is significant because the more we understand its chemical behavior, the more we can apply this understanding for the development of new materials or new technologies, not necessarily only with einsteinium, but also with the rest of actinides. And we can set trends in the periodic table. “
Subjecting their heap disappears to chelated E-254 atoms to X-ray absorption tests and photophysical measurements revealed important details about the bonding distance of the element, while demonstrating wavelength-changing emission behaviors that are not seen in other actinide.
Einsteinium is right on the edge of what we can achieve using basic chemistry. While there are larger elements, their growing circumference puts them at the fingertips of the ability of current technology to create enough for analysis.
But the more we learn about heavy atoms like einsteinium, the greater the potential to find rocks to build giants that are really somewhere on the map.
“Similar to the latest elements that have been discovered in the last 10 years, such as tennessee, which used a target of berkelium, if you could isolate enough pure einsteinium to make a target, you could start looking for other elements and to approach the island of stability (theorized) “, says Abergel.
This research was published in The nature.