If the pandemic has revealed anything, these are the weaknesses of the health system. Lack of technologies, protective equipment, production capacity, shortage problems; the collapse of primary care due to lack of resources and intensive care professionals; vulnerability of surveillance and surveillance structures for epidemic control; the lack of coordination between the autonomous communities, which has led to a slow and heterogeneous response, and the lack of prevention strategies and long-term planning are some of them. But what to do and how to improve management at a critical time?
In December, more than 300 doctors, academics, economists and lawyers published a manifesto proposing the creation of an independent institution to assess health benefits. That is, a public agency with organic status that evaluates and recommends the entry into the system of new drugs, medical devices, tests and diagnostic tests, technologies and surgeries, after a cost-effectiveness analysis by a committee of multidisciplinary experts – specialists, biostatists, health economists, ethicists, patient organizations, epidemiologists, pharmacologists – explains Ruth Puig Peiró and Pilar Pinilla Domínguez, Vice-President and Member of the Health Economics Association (ESA), which organized a follow-up debate, in February. It would be like an Airef, but sanitary, they illustrate.
The idea is to evaluate the cost-effectiveness of all health services, from medicines to medical devices
“In Spain, health management and financing are much more politicized. Decision-making is done from within the ministry [de Sanidad], there is no protection, no guarantees, to minimize such interference, no homogeneous system that can create inequities in access and a significant lack of coordination, “says Puig, who worked at the Health Service of Catalonia (CatSalut ).
The country has the Spanish Network of Health Technology and Benefits Assessment Agencies (Redets), but does not examine the cost of drugs, says Pinilla, which is done through RevalMed. “These agencies exist at the regional level, they are managed as well as they can and they depend a lot on the political management of the moment,” adds Puig. Therefore, they propose the creation of a HispaNice, which imitates the British model, the National Institute for Excellence in Health and Care (Nice), which has been operating since 1999 and whose recommendations are legally binding. In Germany, France, the USA, Canada or Australia, the suggestions of these agencies are not.
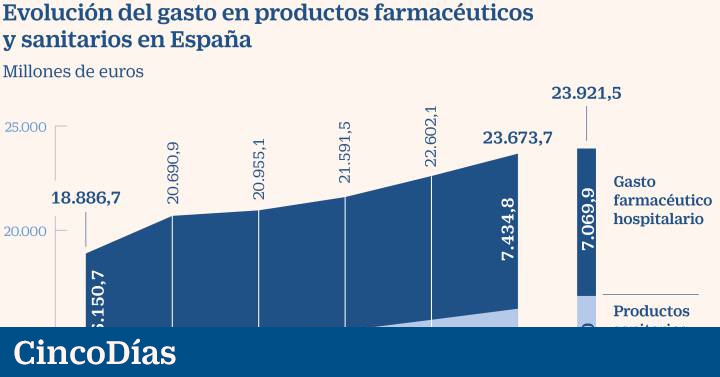
The economist behind the document, Guillem López Casasnovas, a professor at Pompeu Fabra University (UPF) and a member of AES, urges us to work in two areas: streamlining current spending and prioritizing the future. “What has increased in double digits in recent years is pharmaceutical spending in hospitals due to the poorly resolved tension between the hospital pharmacy and clinicians. It is not rationalized which is the best option and which is susceptible to follow-up by the rest of the centers “, he underlines.
And in terms of future spending, he adds: “Someone has to see if the incremental benefit of the new technology, drugs or indications over the existing ones corresponds to the additional costs.” López is in favor of copying the American ICER (Institute for Clinical and Economic Review), which focuses on the analysis of efficacy. “It takes fewer people, you can use the existing network of agencies and you just have to pass a law that protects these actions,” he said. The (uncalculated) investment would be made from European funds and would be self-financed at the rates charged to the industry for its services.
For the employer Farmaindustria, “economic evaluation is necessary in the process of pricing and financing new drugs and the state must decide who performs it. It is important that it is done according to an objective, transparent, participatory and agile procedure ”.
Digitization, another challenge
Digitization of the health system is essential for the incorporation of personalized precision medicines and for patient-centered preventive, diagnostic and therapeutic care. This is stated in a recent report published by the Roche Institute Foundation, which includes 50 recommendations in five areas to advance their implementation, improve management and reduce costs.
The document, led by a group of specialists and experts in various fields, proposes the need to promote technological innovation, to have an interconnected infrastructure and to establish actions to ensure governance and regulation under the values of bioethics.
In addition to guaranteeing the organization, standardization and interoperability of data, their security and improving the training of health professionals, managers and system actors. This will promote, according to the report, a model of preventive care, public health, biomedical research, management and patient participation in decision-making.