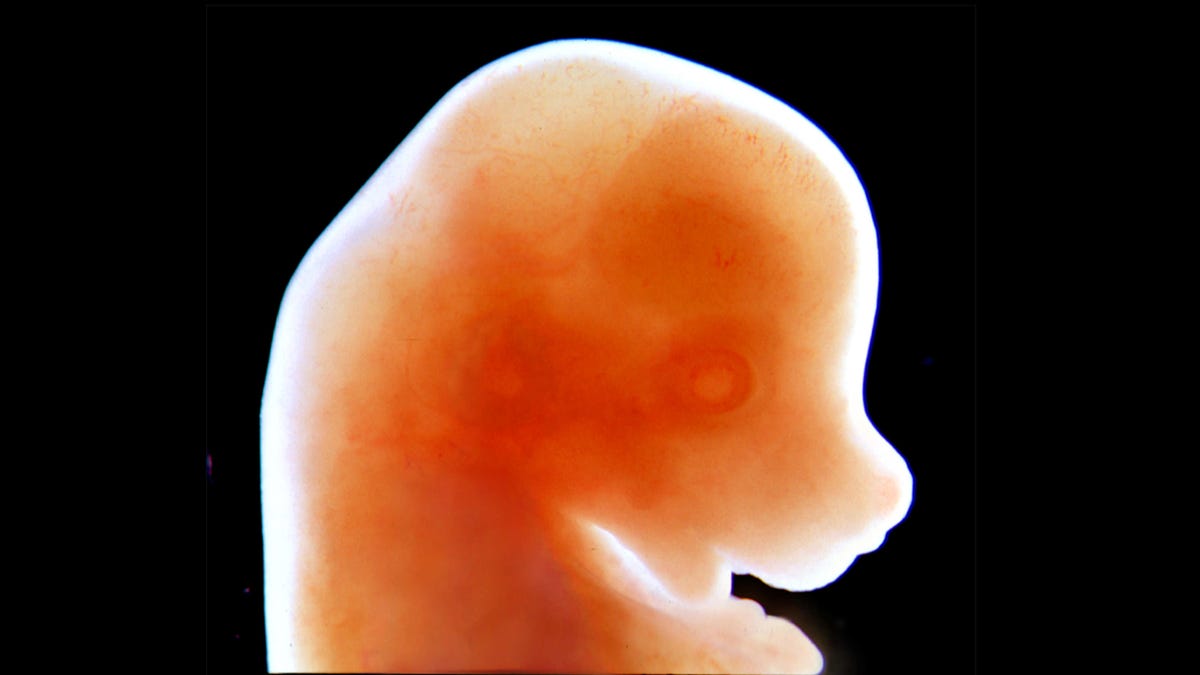
Trembling with life, developing mice moI always see so easily in their ampoules. Just a few days since they were fertilized, rodent embryos They were tiny-smaller than an aspirin tablet“But to them.” their existence is a monumental fact: they developed in an artificial uterus, a first in early mammalian science and a big step in improving scientists’ understanding of embryonic development.
Research, published today, in the journal Nature, he describes how scientists took new embryos and developed them over six days, about a third of the total gestation period of the mouse, outside the rodent uterus.
“If you give the embryo the right conditions, its genetic code will function as a predefined line of dominoes, arranged to fall one after the other,” said co-author Jacob Hanna, a developmental biologist at the Weizmann Institute of Science in Israel, in an Institute of Weizmann science release. “Our goal was to recreate these conditions and now we can watch, in real time, how each domino touches the next in line.”
For almost a century, scientists have invented the idea of taking the embryonic development of mammals outside the uterus to better understand how our cells come together and quickly transform into organisms. Most of the time, however, delving into the early stages of that development was a black box; subsequent stages can be more easily simulated, as they were in 2017, when a group used a bag-like device to incubate lambs on delivery in Philadelphia.
Two years later, the same team has announced could keep premature fetal lambs alive in an artificial womb. After their delivery, premature mammals looked as healthy as their counterparts at the time. “In the world of artificial placenta technology,” said one of the study’s authors at the time, “I actually broke the 4-minute mile.”
Those lambs were much more developed than the newly observed mice. The germinal stages of all mammalian life are difficult to observe in utero, so biologists and geneticists have previously created an idea of what happens by combining observations, such as examining the outer eggs of amphibians and comparing them to images of mouse embryos. dissected. Recent work changes this.
G / O Media may receive a commission
The initial embryos of the mice consisted of only a few hundred cells and were placed on laboratory vessels imitating the uterine wall. After a few days, the team moved the embryos into glasses filled with a nutrient solution and adjusted the amounts of oxygen and carbon dioxide and the pressure of the new embryo environment.ent. After about six days, the growth of the embryo was unsustainable and they were destroyed before the deadline.
There are a few obstacles Hanna hopes to take on: Tthe embryos also needed blood it still had to be fertilized and initially raised in a rodent uterus. In future experiments, Hanna hopes to somehow incorporate artificial blood and synthesize embryos from stem cells to completely avoid the need for a biological uterus.

The new research was published together with another paper in Nature today; that paper describes an early model of human embryo generated from skin cells. The research team was able to reprogram human skin cells into blastocyst-like structures, the embryonic stage that occurs about five days after an egg is fertilized. Synthetic structures, called iBlastoids (as if through a bizarre collaboration between Apple and Pokémon), have significant implications for understanding infertility, the conditions that cause miscarriages, and other aspects of early human development.
“IBlastoids will allow scientists to study the very early stages of human development and some of the causes of infertility, birth defects and the impact of toxins and viruses on early embryos,” said co-author Jose Polo, a developmental biologist at Monash University in Australia. -a university Press release, “Without the use of human blastocysts and, importantly, on an unprecedented scale, accelerating our understanding and development of new therapies.”
Just as tracking race resumes will inform a runner on how to improve their technique, the ability to replicate and observe the primary stages of mammalian life several times will help scientists understand how to improve living conditions in the beginning.